Comparative analysis of delivery systems and their impact on tear film lipid layer stability, linking formulation design to ocular surface behavior.

Safer and smarter eye formulations powered by next-generation in vitro and in silico tear film and cornea models.
Specialized in vitro and in silico testing for safer ophthalmic and cosmetic formulations.
Eyveris provides mechanistic, acellular tear film and cornea models to evaluate ocular compatibility, irritability, and formulation behavior. We work with pharma and cosmetics partners to enable safer, more predictable product development and reduce R&D risk without relying on standard cellular and animal models.
Mechanistic, acellular interface platforms—complemented by molecular simulations—to de-risk ocular products early.
Under the hood →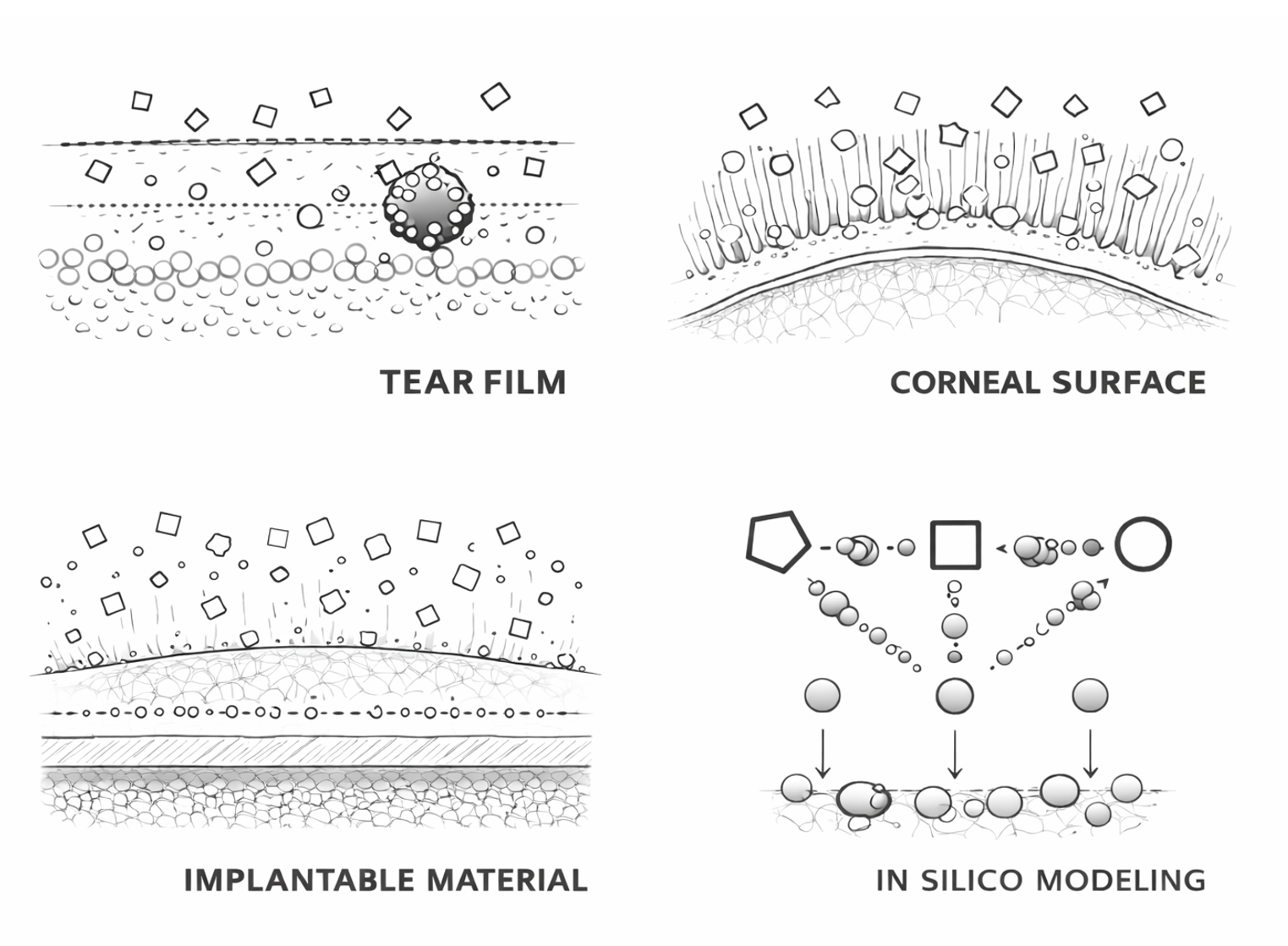



About

Tear film & cornea–driven ocular formulation testing
Eyveris is a research-driven startup specializing in tear film and cornea models for mechanistic assessment of ocular irritation, compatibility, and formulation performance. Our technologies are built on long-term basic research in tear film biophysics, corneal surface science, and interfacial phenomena, as well as on collaborative projects with pharmaceutical partners.
Beyond the experimental models themselves, we develop specialized data analysis methodologies, proprietary software tools, and standardized workflows that ensure reproducible and interpretable results. This allows us to operate under strict academic standards while meeting the robustness expected in industrial and regulatory settings.
We collaborate with pharmaceutical and cosmetics companies to de-risk product development, support regulatory strategies, and accelerate innovation in ophthalmic and periocular formulations.
Services
What we offer
Integrated testing and R&D support based on tear film and cornea models, tailored to ophthalmic and periocular products.
- Tear film destabilization and breakup analysis
- Differentiation of formulation irritation potential
- Fully in vitro, animal-free methodologies
- Mechanistic insight into formulation–tear film and cornea interactions
- Excipients and preservative strategy
- Guidance for stability, tolerability, and performance
- Polymeric implantable materials and contact lenses
- Contact lens cleaning and storage solutions
- Microplastic by-products from cosmetics and packaging materials
From early screening to comparative studies, we provide mechanistic data that support safer, more robust product decisions.
Platform
Our integrated in vitro & in silico models
We combine proprietary acellular tear film and cornea models with molecular-resolution simulations to capture how formulations interact with the ocular surface at multiple scales. These models are supported by dedicated analysis pipelines, custom software, and standardized workflows that help convert complex data into actionable insight.
Under the hood
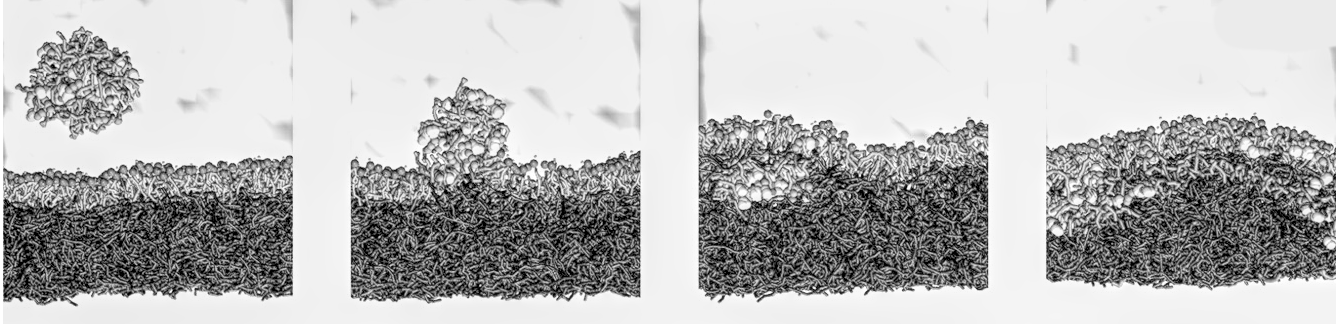
Our tear film interface combines an aqueous phase with a lipidomics-guided complex lipid multilayer, enabling mechanistic assessment of tear-layer interactions.
Our corneal platform is a glycocalyx-relevant, acellular corneal-interface mimic, built to reflect key interfacial features that govern irritation-relevant interactions.
Our computational workflow integrates coarse-grained and atomistic molecular dynamics (MD) simulations to explore molecular and nanoscale mechanisms.
- Destabilisation and disruption of tear layers
- Drug partitioning at the tear lipid layer, aqueous layer and corneal surface
- In situ decomposition of nanocarriers
- Biologically and medicinally relevant acellular tear film and cornea models
- Real-time tear film destabilization and breakup analysis
- Structural changes at the ocular interface in the presence of formulations
- Simulated blinking cycles
- Molecular-resolution tear film and cornea simulations
- Drug–lipid and excipient–tear lipid layer interactions
- Drug nanocarrier passage and decomposition mechanisms
- Mechanistic insight and quantitative evaluation for rational formulation design and troubleshooting
The first integrated in vitro + in silico platform dedicated to ocular formulation testing, combining advanced tear film and cornea models with specialized data analysis and standardized workflows.
Applications
Where we can help
We support both targeted irritation testing and broader formulation optimization across ophthalmic and cosmetic pipelines.
- Artificial tears and OTC lubricants
- Prescription eye drops (e.g., glaucoma, dry eye)
- Nanocarriers, preservatives, and excipients
- Ocular drug delivery systems
- Cosmetics with ocular or periocular exposure
- Artificial eyelashes, glues, and removers
- Independent verification of low-irritation claims
- Benchmarking against competitor products
Reference work
Selected research background
Our methods are supported by peer-reviewed research on tear film, cornea, and ocular surface science, including both experimental and computational work carried out in academic settings and in collaboration with pharmaceutical partners.
Mechanistic evaluation of preservative effects on tear film lipid layer structure and function, informing safer preservative strategies.
In vitro and in silico studies on drug integration into tear film lipids, revealing molecular-level interactions relevant for tolerability.
Biocompatibility assessment of polymers used in ophthalmic implants and devices, focusing on their interaction with ocular lipid layers.
Collaboration
Flexible collaboration models
From quick comparative studies to integrated R&D programs, we adapt our platform, analysis workflows, and reporting standards to your development stage and regulatory needs while maintaining academic-level scientific rigor.
- Tear film irritation and stability testing
- Comparative screening of formulations or materials
- Certification support for low-irritation cosmetics
- Formulation development and optimization
- Mechanistic R&D and root-cause analysis
- Rational design of drug nanocarrier systems
- Integrated in vitro + in silico pipelines
Team & contact
Work with us
Our team combines expertise in tear film biophysics, corneal surface science, molecular simulations, formulation development, and materials testing. We are experienced in delivering projects that meet both academic publication standards and industrial quality and documentation requirements.
Our expertise
- Tear film and cornea biophysics
- Surface and interface science
- Molecular simulations and in silico model development
- Formulation chemistry and ocular delivery
- Biocompatibility and materials testing
- Specialized data analysis and proprietary software tools